Johnson and Johnson COVID Vaccine Rollout Stopped After Reports of Blood Clots
-
The United States and European Union will temporarily stop the rollout of the Johnson and Johnson COVID vaccine after reports of blood clots
-
According to the US Food and Drug Administration (FDA), six cases of blood clot were reported among 6.8 million people vaccinated
-
J&J vaccine is the second vaccine to cause blood clots after similar incidents with the AstraZeneca vaccine
The US, South Africa, and the European Union have all stopped the rollout of the Johnson and Johnson COVID vaccine after reported cases of blood clots.
According to the US Food and Drug Administration (FDA), 6 cases of blood clot was reported among 6.8 million people that received the vaccine.
The J&J blood clot complications follow similar issues that were recorded during the use of the AstraZeneca vaccine.
The FDA recommended the pause of the vaccine after a patient died from blood clot complications. Another is also reported to be in critical condition.
All six cases of blood clot complication were recorded among women between the ages of 18 to 48.
The complications also came up within 13 days after the vaccination.
Nigeria recently announced that it had plans to receive 70 million doses of the Johnson & Johnson vaccine.
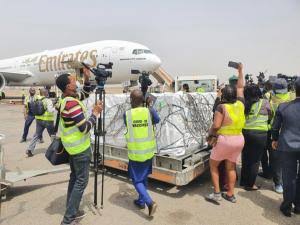
It is yet unknown if the country will go ahead with the rollout of the vaccine following this information.
Read also: Nigeria set to create its own COVID vaccine
Nigeria is currently using the AstraZeneca vaccines which were received in March.
Follow Naija.fm on our social media handles Facebook, Instagram, and Twitter to keep up with trending news, breaking news, entertainment news, and hot gist.


